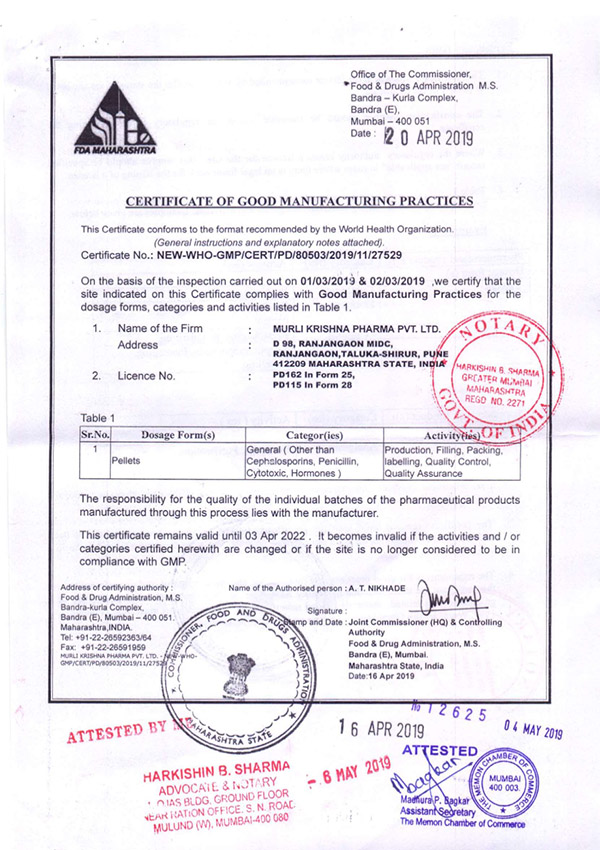
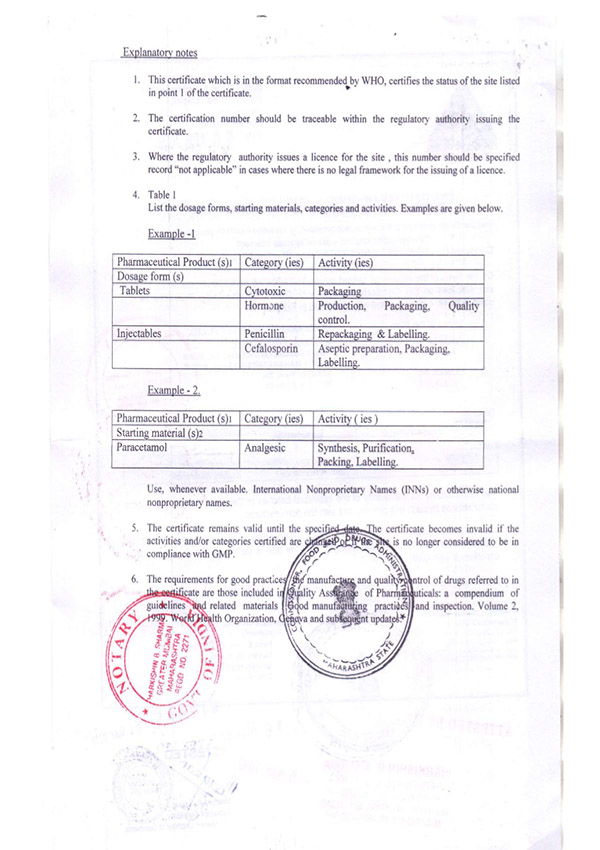
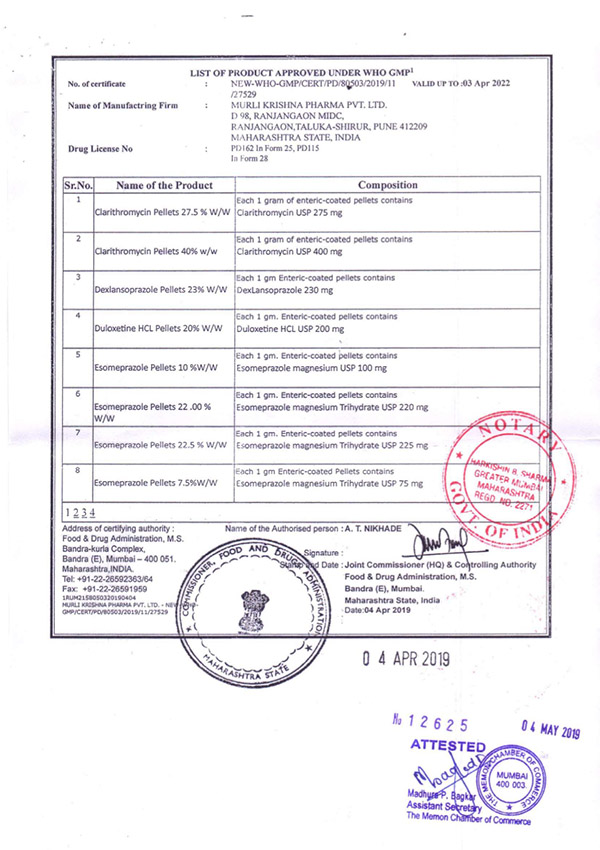
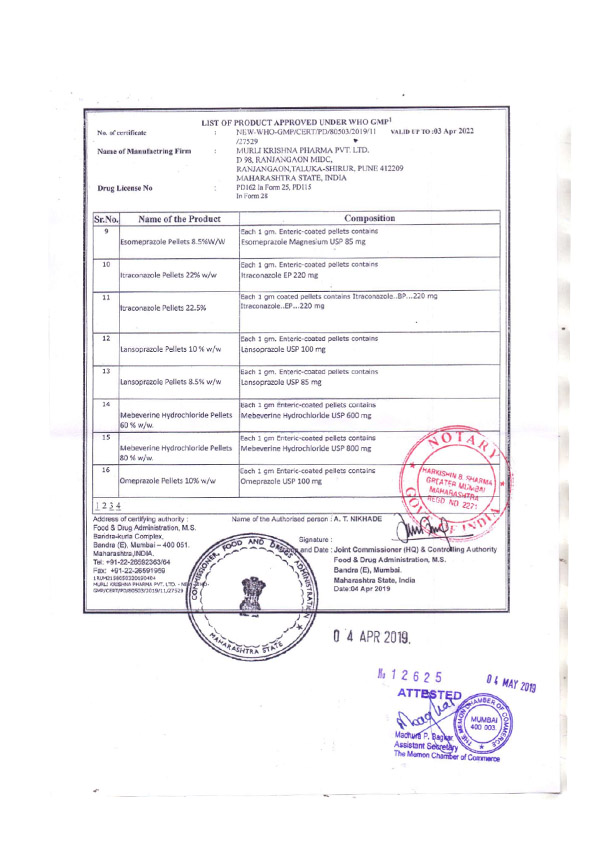
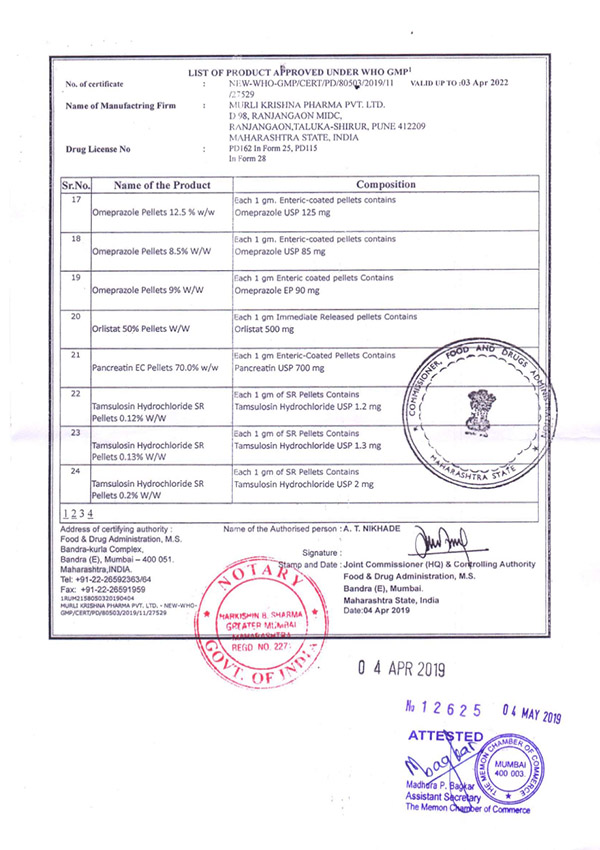
Regulatory Approvals and Audits
Murli Krishna Pharma proudly maintains a robust record of regulatory compliance, bolstered by a series of esteemed approvals from various international health authorities. Our commitment to quality and excellence has earned us certifications from the European Union, Ministry of Health UAE, Jordanian FDA, WHO (India), FDA (India), and the Central Drugs Standard Control Organization (CDSCO). These approvals affirm our adherence to stringent global standards and reinforce our reputation as a trusted player in the pharmaceutical industry.
In addition to these prestigious recognitions, we have successfully navigated audits from several key generic pharmaceutical companies, demonstrating our readiness to meet the rigorous demands of the marketplace. Furthermore, we are currently awaiting regulatory audits from the US FDA, Saudi Arabia, and MHRA, reflecting our ongoing commitment to expanding our global footprint and meeting diverse regulatory requirements.
Our R&D Lab has received approval from the Department of Scientific and Industrial Research (DSIR), Government of India, signifying our dedication to fostering innovative research and development. This recognition underscores our strategic focus on advancing cutting-edge solutions, ensuring we remain at the forefront of pharmaceutical advancements.
At MKPPL, we understand the critical importance of regulatory compliance in ensuring product safety and efficacy. As such, we continually strive to enhance our processes and uphold the highest standards in our operations. We are excited about the opportunities ahead and remain committed to delivering innovative, high-quality products to our customers around the globe.